Скачать с ютуб Iron Carbon phase Diagram Part 1 в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Iron Carbon phase Diagram Part 1 в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Iron Carbon phase Diagram Part 1 или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Iron Carbon phase Diagram Part 1 в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Iron Carbon phase Diagram Part 1
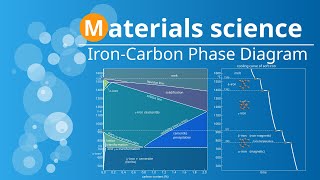
An iron-carbon phase diagram is a vital tool in understanding the behavior of iron-carbon alloys during heat treatment. By examining this diagram, we can gain insights into the different phases that form at varying temperatures and carbon concentrations. Phases in Iron Carbon Phase Diagram Ferrite phase Austenite Phase Delta Iron Cementite Phase Pearlite phase Ledeburite phase Ferrite Phase The Ferrite phase is an Interstitial Solid solution of carbon in alpha iron that has a Body-Centered cubic crystal structure. Interstitial solid solutions are solid-state solutions that form when solute atoms occupy the interstitial spaces. In this ferrite phase, carbon atoms are the solute atoms and they occupy the interstitial spaces between the iron atoms. Solubility Limit of Ferrite phase The maximum solubility limit of dissolving carbon into the ferrite phase is only 0.025 Wt% at 723C. But this solubility limit of dissolving carbon in the iron phase keeps on decreasing up to 0.00005% as we cool it down to room temperature. And these Carbon atoms preferentially sit in the tetrahedral interstitial site of the BCC unit cell in this ferrite phase. Austenite Phase The Austenite phase is also an interstitial solid solution of carbon in fcc iron and in this interstitial solid solution, carbon atoms occupy the octahedral interstitial positions rather than tetrahedral voids. Due to this reason, the solubility limit of dissolving carbon into the fcc unit cell of this austenite phase is greater than the ferrite phase. Solubility Limit of Austenite Phase This Austenite phase can dissolve up to 2.03% of Carbon into its FCC matrix at 1147C. Delta Phase If we slowly cool down the pure iron below its melting point (1539 °C) it will crystallize into a phase, which has a body-centered cubic (BCC) crystal structure. This phase is known as the delta iron. The BCC crystal structure of this delta phase is different from the BCC crystal structure of the alpha phase that exists at room temperature because the BCC crystal structure of the delta phase has larger lattice parameters as compared to the BCC crystal structure of the ferrite phase. This delta phase is also an interstitial solid solution of C in bcc iron but it exist in the high temperature region of the diagram. This phase is only stable at temperature above 1400 °C and starts melting at temperature above 1539 °C. Because of the larger lattice parameters of this bcc phase its solubility limit of dissolving carbon into its lattice is greater than the alpha phase. So the delta-iron can dissolve as much as 0.08% of carbon in its interstitial solid solution at 1,492 °C This channel will provide you with the complete heat treatment course explained through PowerPoint animation. #heattreatment #materialscience #metallurgical #metallurgicalengineering #metallurgy #steel





![What is Physical Metallurgy Lecture 1 Part 1 [Level 1 Course]](https://i.ytimg.com/vi/TUrzti3Y4pg/mqdefault.jpg)


