Скачать с ютуб Iron Carbon Phase Diagram Part 2 | Heat Treatment Course в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Iron Carbon Phase Diagram Part 2 | Heat Treatment Course в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Iron Carbon Phase Diagram Part 2 | Heat Treatment Course или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Iron Carbon Phase Diagram Part 2 | Heat Treatment Course в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Iron Carbon Phase Diagram Part 2 | Heat Treatment Course
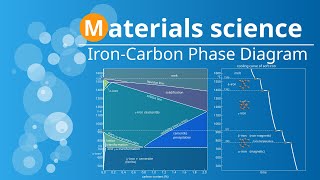
Cementite, Pearlite and Ledeburite Phases Cementite is an stoichiometric intermetallic compound that consists of iron and carbon atoms. Stoichiometric intermetallic compounds are chemical compounds that usually contain two or more metallic elements. These compounds have specific stoichiometric ratios, meaning the elements combine in precise proportions. That is why the intermetallic compounds are represented by a vertical line on the phase diagram where the composition is fixed. This cementite phase consists of iron and carbon atoms in a fixed ratio of 6.67:1. Which means that cementite is formed at 6.67 wt% of C. Its crystallographic unit cell is orthorhombic and primitive, with large lattice parameters, explaining its hardness. In this type of crystal structure, the lattice consists of three axes that are perpendicular to each other and unequal in length. This phase is an incredibly hard and brittle phase. Its hardness makes it one of the primary components responsible for the hardness of steel. In fact, cementite is one of the hardest constituents in steel alloys. In the iron-carbon phase diagram, the cementite phase is formed through four different reactions, each occurring at different compositions. Let's explore each of these reactions one by one. The cementite phase can be formed through liquid-to-solid state transformation. For example, when the iron-carbon liquid alloy of hypereutectic composition solidifies, cementite forms as primary particles in the remaining liquid. As the solidification process continues, the remaining liquid solidifies into austenite or ferrite, depending on the cooling rate and alloy composition. This type of transformation is known as liquid-to-solid phase transformation. Similarly, Cementite can also be formed through solid-to-solid state transformations, where one solid phase transforms into another without involving any liquid phase. Let's consider the example of hyper eutectoid steel with a carbon content above the eutectoid composition. When this hypereutectoid steel is cooled from the austenitic region, the excess carbon will diffuse and combine with the existing austenite. As a result, cementite networks form, creating interconnected structures within the remaining austenite matrix. This cementite network significantly influences the steel's strength and wear resistance. The cementite phase can also be formed by the eutectoid reaction. A eutectoid reaction is a type of phase transformation that involves three phases. During this reaction, as a solid material undergoes cooling, it simultaneously transforms into two distinct solid phases. In the iron-carbon phase diagram, the eutectoid reaction results in the formation of the pearlite phase from the austenite phase. This pearlite phase consists of alternating layers of ferrite and cementite that give pearlite its distinctive lamellar or pearly appearance when observed under the microscope. This microstructure contributes to the mechanical properties of the steel, such as its strength and hardness. The eutectoid reaction is also an invariant reaction which occurs at constant temperature and composition. Its reaction mechanism will be also discussed in this lecture series. The formation of the cementite phase can also occur through a process known as a eutectic reaction. In a eutectic reaction, a liquid solution undergoes a simultaneous transformation into two solid phases as it cools down. This phenomenon is characterized by its invariance, which means it happens at a constant temperature and composition. In the iron-carbon phase diagram this eutectic reaction results in the formation of the ledeburite phase which is a eutectic mixture of austenite and cementite. So in this eutectic reaction, the liquid phase with 4.3 %C at a fixed temperature of 1147oC gives two solid phases i.e., Austenite and Cementite upon cooling. Its mechanism will be discussed in the upcoming lecture. The ledeburite phase can appear with any composition between 2.06% and 6.67% of carbon. This phase is characterised by its distinctive appearance under the microscope, showing regions of dark cementite embedded in a matrix of bright austenite. It is a brittle phase typically undesirable in most applications due to its poor mechanical properties. #metallurgy #metallurgicalengineering #metallurgical #ironcarbonphasediagram #heattreatment #cementite #physicalmetallurgy