Скачать с ютуб Medical Devices in the EU Overcoming Challenges and Securing CE Mark в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Medical Devices in the EU Overcoming Challenges and Securing CE Mark в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Medical Devices in the EU Overcoming Challenges and Securing CE Mark или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Medical Devices in the EU Overcoming Challenges and Securing CE Mark в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Medical Devices in the EU Overcoming Challenges and Securing CE Mark
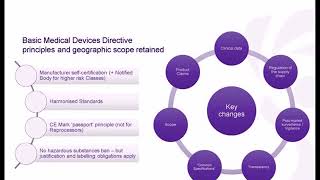
Galen Data is honored to have been joined by Dan Stoller of CENIT Consulting for a insightful discussion on MedTech go-to-market in Europe. For any medical device manufacturer, getting an approval from a Notified Body or regulatory agency can be expensive and time-consuming. It’s become especially difficult in the EU over the past couple of years due to the shift from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR). In this webinar, we cover some of the reasons why and try to give you insights into the challenges of submitting for a CE Mark for the MDR and how to manage those challenges. Key Takeaways: • Understanding the shift from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) in the EU and its implications for medical device manufacturers. • Insights into the challenges and complexities involved in obtaining a CE Mark for the MDR, including the approval process with Notified Bodies and regulatory agencies. • Strategies and best practices for managing the expenses and time-consuming aspects of submitting for a CE Mark, enabling a smoother MedTech go-to-market in Europe.