РЎРәР°СҮР°СӮСҢ СҒ СҺСӮСғРұ Protein folding | tertiary and quaternary structure #8 ШҙШұШӯ ШӘШ·ШЁЩҠЩӮ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶ РІ С…РҫСҖРҫСҲРөРј РәР°СҮРөСҒСӮРІРө
РЎРәР°СҮР°СӮСҢ РұРөСҒРҝлаСӮРҪРҫ Рё СҒРјРҫСӮСҖРөСӮСҢ СҺСӮСғРұ-РІРёРҙРөРҫ РұРөР· РұР»РҫРәРёСҖРҫРІРҫРә Protein folding | tertiary and quaternary structure #8 ШҙШұШӯ ШӘШ·ШЁЩҠЩӮ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶ РІ РәР°СҮРөСҒСӮРІРө 4Рә (2Рә / 1080p)
РЈ РҪР°СҒ РІСӢ РјРҫР¶РөСӮРө РҝРҫСҒРјРҫСӮСҖРөСӮСҢ РұРөСҒРҝлаСӮРҪРҫ Protein folding | tertiary and quaternary structure #8 ШҙШұШӯ ШӘШ·ШЁЩҠЩӮ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶ или СҒРәР°СҮР°СӮСҢ РІ РјР°РәСҒималСҢРҪРҫРј РҙРҫСҒСӮСғРҝРҪРҫРј РәР°СҮРөСҒСӮРІРө, РәРҫСӮРҫСҖРҫРө РұСӢР»Рҫ загСҖСғР¶РөРҪРҫ РҪР° СҺСӮСғРұ. ДлСҸ СҒРәР°СҮРёРІР°РҪРёСҸ РІСӢРұРөСҖРёСӮРө РІР°СҖРёР°РҪСӮ РёР· С„РҫСҖРјСӢ РҪРёР¶Рө:
ЗагСҖСғР·РёСӮСҢ РјСғР·СӢРәСғ / СҖРёРҪРіСӮРҫРҪ Protein folding | tertiary and quaternary structure #8 ШҙШұШӯ ШӘШ·ШЁЩҠЩӮ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶ РІ С„РҫСҖРјР°СӮРө MP3:
Р•СҒли РәРҪРҫРҝРәРё СҒРәР°СҮРёРІР°РҪРёСҸ РҪРө
загСҖСғзилиСҒСҢ
РқРҗР–РңРҳРўР• ЗДЕСЬ или РҫРұРҪРҫРІРёСӮРө СҒСӮСҖР°РҪРёСҶСғ
Р•СҒли РІРҫР·РҪРёРәР°СҺСӮ РҝСҖРҫРұР»РөРјСӢ СҒРҫ СҒРәР°СҮРёРІР°РҪРёРөРј, РҝРҫжалСғР№СҒСӮР° РҪР°РҝРёСҲРёСӮРө РІ РҝРҫРҙРҙРөСҖР¶РәСғ РҝРҫ Р°РҙСҖРөСҒСғ РІРҪРёР·Сғ
СҒСӮСҖР°РҪРёСҶСӢ.
РЎРҝР°СҒРёРұРҫ Р·Р° РёСҒРҝРҫР»СҢР·РҫРІР°РҪРёРө СҒРөСҖРІРёСҒР° savevideohd.ru
Protein folding | tertiary and quaternary structure #8 ШҙШұШӯ ШӘШ·ШЁЩҠЩӮ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶ
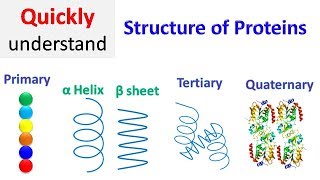
Zewail City OCW channel рҹ”Ҫ В В В /В @zcocwВ В Dr. Reem's Biochemistry Course not available yet Description: ШӘШӘШ¬Щ…Ш№ Ш§Щ„ШіЩ„Ш§ШіЩ„ Ш§Щ„Ш«Ш§ЩҶЩҲЩҠШ© ШЈЩ„ЩҒШ§ ЩҲШЁЩҠШӘШ§ Щ…Ш№ ШЁШ№Ш¶ЩҮШ§ ШӘШ§ШұЩғШ© ШЁЩҠЩҶЩҮШ§ ЩҶЩӮШ§Ш· Ш§ЩҶШ№Ш·Ш§ЩҒ ШЁЩҲШ§ШіШ·Ш© Щ…Ш¬Щ…ЩҲШ№Ш© Щ…ЩҶ Ш§Щ„ШұЩҲШ§ШЁШ·ШҢ ШЈЩҮЩ…ЩҮШ§ Ш«ЩҶШ§ШҰЩҠШ© Ш§Щ„ЩғШЁШұЩҠШӘ ЩҲШ§Щ„ЩғШ§ШұЩҮШ© Щ„Щ„Щ…Ш§ШЎШҢ Щ„ШӘШ№Ш·ЩҠ Ш§Щ„ШЁЩҶЩҠШ© Ш§Щ„Ш«Ш§Щ„Ш«ЩҠШ© ШЈЩҲ Ш§Щ„ШӘШӯШӘ ЩҲШӯШҜШ©. ШӘШӘШ¬Щ…Ш№ Ш§Щ„ШӘШӯШӘ ЩҲШӯШҜШ§ШӘ Щ„ШӘШ№Ш·ЩҠ ШЁШұЩҲШӘЩҠЩҶ Ш°ЩҲ ШЁЩҶЩҠШ© ШұШ§ШЁШ№ЩҠШ© ЩҲШёЩҠЩҒЩҠ. ЩҲЩ„ЩғЩ„ ШЁШұЩҲШӘЩҠЩҶ ШӘШіЩ„ШіЩ„ Щ„Щ„ШЈШӯЩ…Ш§Ш¶ Ш§Щ„ШЈЩ…ЩҠЩҶЩҠШ© Ш®Ш§Шө ШЁЩҮ ЩҒЩӮШ·ШҢ ЩҲЩҠЩҶШӘЩҮЩҠ ШЁШ·ШұЩҠЩӮШ© Щ…Ш§ШҢ ШЁШ§Щ„Ш·ЩҠЩ‘Ш© Ш§Щ„Щ…Щ…ЩҠШІШ© Щ„ЩҮ. Щ„ЩғЩҶ Щ…Ш«Щ„ ЩҮШ°ЩҮ Ш§Щ„ШіЩ„ШіЩ„Ш© Ш§Щ„Щ…Ш№ЩӮШҜШ© ЩҠЩ…ЩғЩҶ ШЈЩҶ ШӘЩҶШ·ЩҲЩҠ ШЁШ№ШҜШҜ ЩғШЁЩҠШұ Ш¬ШҜШ§ЩӢ Щ…ЩҶ Ш§Щ„Ш·ШұЩӮ Ш§Щ„Щ…Ш®ШӘЩ„ЩҒШ©ШҢ ЩҲЩ…ЩҶ Ш«Щ… ЩғЩҠЩҒ ЩҠШӘШЈШӘЩү Щ„Щ„ШЁШұЩҲШӘЩҠЩҶ ШЈЩҶ ЩҠЩҶШӘЩҮЩҠ ШЁШ§Щ„ШҙЩғЩ„ Ш§Щ„ШөШӯЩҠШӯ Щ„ЩҮ ШЁШ§Щ„Ш¶ШЁШ·? Щ„Ш§ ЩҠЩ…ЩғЩҶ Ш§Щ„ШҘШ¬Ш§ШЁШ© Ш№ЩҶ ЩҮШ°Ш§ Ш§Щ„ШіШӨШ§Щ„ ШЁШ§Щ„ШӘШ®Щ…ЩҠЩҶ ШЈЩҲ ШЁШ§ШӘШЁШ§Ш№ Ш·ШұЩҠЩӮШ© Ш§Щ„ШӘШ¬ШұШЁШ© ЩҲШ§Щ„Ш®Ш·ШЈ. ШҘЩҶ Ш№Щ…Шұ Ш§Щ„ЩғЩҲЩҶ ЩҶЩҒШіЩҮ ЩҠШ№ШҜ ЩӮШөЩҠШұШ§ЩӢ Щ…ЩӮШ§ШұЩҶШ© ШЁШ§Щ„ЩҲЩӮШӘ Ш§Щ„Ш°ЩҠ ЩҠШіШӘШәШұЩӮЩҮ ШЁШұЩҲШӘЩҠЩҶ ШөШәЩҠШұ Щ„ШӘШ¬ШұШЁШ© ШЁЩ„Ш§ЩҠЩҠЩҶ Щ…ЩҶ Ш§Щ„Ш·ЩҠЩ‘Ш§ШӘ Ш§Щ„Щ…Щ…ЩғЩҶШ© ЩҲШ§ШӯШҜШ© ЩҲШұШ§ШЎ ШЈШ®ШұЩүШҢ ЩҲШөЩҲЩ„Ш§ЩӢ ШҘЩ„Щү Ш§Щ„Ш·ЩҠЩ‘Ш© Ш§Щ„ШөШӯЩҠШӯШ©! ШҘШ° ШҘЩҶ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶШ§ШӘ ШӘЩҶШ«ЩҶЩҠ ЩҲШӘЩ„ШӘЩҲЩҠ ЩҲШӘЩ„ШӘЩҒ ЩҒЩҠ ШҙЩғЩ„ ШӯЩ„ЩӮШ§ШӘ ШЈЩҲ ШӯЩ„ШІЩҲЩҶШ§ШӘШҢ ШЁЩҠЩҶЩ…Ш§ ШӘЩҶШ¶ШәШ· ШЁШ№Ш¶ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶШ§ШӘ Ш§Щ„ШЈШ®ШұЩү ЩҒЩҠ ШұЩӮШ§ШҰЩӮ Щ…Ш·ЩҲЩҠЩ‘Ш© ШӘШҙШЁЩҮ Ш§Щ„ШўЩ„Ш© Ш§Щ„Щ…ЩҲШіЩҠЩӮЩҠШ© (Ш§Щ„ШЈЩғЩҲШұШҜЩҠЩҲЩҶ)ШҢ ЩҲЩғШ°Щ„Щғ ЩҒЩҠ ШЈШҙЩғШ§Щ„ ШЈШ®ШұЩү. ЩҲЩҮШ°ЩҮ Ш§Щ„Ш·ЩҠЩ‘Ш§ШӘ ШӘШіШ§Ш№ШҜ Ш№Щ„Щү ШЈШҜШ§ШЎ Ш§Щ„ШЁШұЩҲШӘЩҠЩҶШ§ШӘ Щ„ЩҲШёШ§ШҰЩҒЩҮШ§ Ш§Щ„Ш¬ЩҲЩҮШұЩҠШ© ШҜШ§Ш®Щ„ Ш§Щ„Ш®Щ„Ш§ЩҠШ§. Tertiary structure The overall three-dimensional structure of a polypeptide is called its tertiary structure. The tertiary structure is primarily due to interactions between the R groups of the amino acids that make up the protein. R group interactions that contribute to the tertiary structure include hydrogen bonding, ionic bonding, dipole-dipole interactions, and London dispersion forces вҖ“ basically, the whole gamut of non-covalent bonds. For example, R groups with like charges repel one another, while those with opposite charges can form an ionic bond. Similarly, polar R groups can form hydrogen bonds and other dipole-dipole interactions. Also important to a tertiary structure are hydrophobic interactions, in which amino acids with nonpolar, hydrophobic R groups cluster together on the inside of the protein, leaving hydrophilic amino acids on the outside to interact with surrounding water molecules. Finally, thereвҖҷs one special type of covalent bond that can contribute to tertiary structure: the disulfide bond. Disulfide bonds, covalent linkages between the sulfur-containing side chains of cysteines, are much stronger than the other types of bonds that contribute to tertiary structure. They act like molecular "safety pins," keeping parts of the polypeptide firmly attached to one another. Quaternary structure Many proteins are made up of a single polypeptide chain and have only three levels of structure (the ones weвҖҷve just discussed). However, some proteins are made up of multiple polypeptide chains, also known as subunits. When these subunits come together, they give the protein its quaternary structure. WeвҖҷve already encountered one example of a protein with a quaternary structure: hemoglobin. As mentioned earlier, hemoglobin carries oxygen in the blood and is made up of four subunits, two each of the Оұ and ОІ types. Another example is DNA polymerase, an enzyme that synthesizes new strands of DNA and is composed of ten subunits . In general, the same types of interactions that contribute to tertiary structure (mostly weak interactions, such as hydrogen bonding and London dispersion forces) also hold the subunits together to give quaternary structure. Denaturation and protein folding Each protein has its own unique shape. If the temperature or pH of a protein's environment is changed, or if it is exposed to chemicals, these interactions may be disrupted, causing the protein to lose its three-dimensional structure and turn back into an unstructured string of amino acids. When a protein loses its higher-order structure, but not its primary sequence, it is said to be denatured. Denatured proteins are usually non-functional. For some proteins, denaturation can be reversed. Since the primary structure of the polypeptide is still intact (the amino acids havenвҖҷt split up), it may be able to re-fold into its functional form if it's returned to its normal environment. Other times, however, denaturation is permanent 0:10 Revision 0:29 Super 2ndry structure 1:38 Domains 2:30 3ry structure 6:45 4ry structure 7:10 Bonus station рҹ“ЈЩ„Щ„ШӘЩҲШ§ШөЩ„рҹ“Ј ШөЩҒШӯШӘЩү Ш§Щ„ШҙШ®ШөЩҠШ©: В В /В radwan.derbala.57357В В ШөЩҒШӯШ© Щ…ШіШ§ЩҒШ© Ш§Щ„ШіЩғШ©: https://www.facebook.com/%D9%85%D8%B3... LinkedIn: В В /В 56408994В В Щ„Щ„ШӘЩҲШ§ШөЩ„ Щ…Ш№ ШӘШ№Щ„Щ… Ш§ЩҲЩҶ Щ„Ш§ЩҠЩҶ: В В В /В animationineducationofficialВ В В В В /В @-cramontheroad8584В В follow us on Facebook: Facebook.com/Animation.edu #Animation_in_Education #Ta3allam #ШӘШ№Щ„Щ‘Щ…