Скачать с ютуб What is the design control process and how has it changed? в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок What is the design control process and how has it changed? в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно What is the design control process and how has it changed? или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон What is the design control process and how has it changed? в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
What is the design control process and how has it changed?
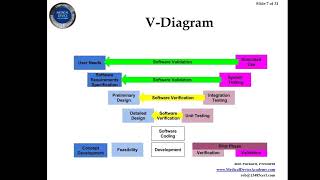
The FDA first mandated that medical device manufacturers implement design controls in 1996. The process was copied from Health Canada and the ISO 9001:1994 Standard. Today, people are still teaching the “Waterfall” diagram, but the design of medical devices was never a linear process. The V-diagram from IEC 62304 is closer to the real design control process, but even that process is oversimplified. The design control process is the collection of methods used by a team of people and companies to ensure that a new medical device will meet the requirements of customers, regulators, recognized standards, and stakeholders. The design control process must integrate the disciplines of risk management and human factors. Process success is verified by conducting the verification and validation testing, while the process ends when the team agrees that all of the design transfer activities are completed and regulatory approval is received. Timestamps: 0:00 What are Design Controls? 1:44 The V-Diagram 2:04 The problem with the design control process 3:50 User Needs 5:19 The Design Inputs 7:27 The Design Freeze 8:49 Verification & Validation (V&V) Testing 9:36 Electrical Safety 11:36 Iterative Process 13:13 Definition of Design Controls 17:56 Cybersecurity Requirements 21:36 How to you maintain your DHF? 22:31 Design History File