Скачать с ютуб FDA Approves Atezolizumab / Tecentriq Hybreza™️ For Subcutaneous PD-L1 Cancer Immunotherapy in NSCLC в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок FDA Approves Atezolizumab / Tecentriq Hybreza™️ For Subcutaneous PD-L1 Cancer Immunotherapy in NSCLC в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно FDA Approves Atezolizumab / Tecentriq Hybreza™️ For Subcutaneous PD-L1 Cancer Immunotherapy in NSCLC или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон FDA Approves Atezolizumab / Tecentriq Hybreza™️ For Subcutaneous PD-L1 Cancer Immunotherapy in NSCLC в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
FDA Approves Atezolizumab / Tecentriq Hybreza™️ For Subcutaneous PD-L1 Cancer Immunotherapy in NSCLC
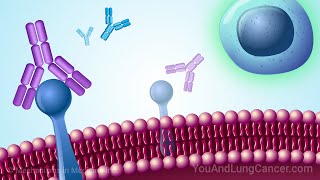
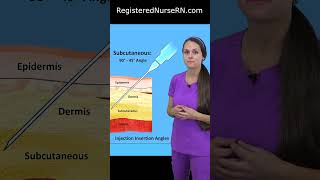
More Info: https://oncologytube.com/video-fda-ap... In this video, we explore the FDA approval of Genentech’s Tecentriq Hybreza, the first subcutaneous anti-PD-(L)1 immunotherapy for cancer. Approved on September 12, 2024, this new formulation allows patients to receive Tecentriq via a 7-minute injection under the skin, offering a faster, more convenient alternative to the traditional intravenous (IV) infusion, which takes 30-60 minutes. This advancement provides greater flexibility for both patients and healthcare providers. Tecentriq Hybreza is approved for the same indications as IV Tecentriq, making it an option for patients with various types of cancer, including non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma (HCC), and others. By reducing treatment time, Tecentriq Hybreza enhances the patient experience, allowing for more comfortable, time-efficient cancer care. Key points covered in the video include: Data from the Phase IB/III IMscin001 study demonstrated that Tecentriq Hybreza achieved comparable blood levels and efficacy to the IV formulation, with a similar safety profile. The Phase II IMscin002 study showed that 71% of patients preferred the subcutaneous injection due to reduced clinic time, greater comfort during treatment, and decreased emotional distress. Additionally, 79% of patients who experienced both forms of Tecentriq chose to continue with the subcutaneous option. Tecentriq Hybreza is already approved in over 50 countries, continuing to expand access to faster cancer treatment worldwide. By offering patients a more flexible, efficient way to receive their cancer treatment, Tecentriq Hybreza represents a major leap forward in cancer immunotherapy. This new administration method maintains the same efficacy as the IV version while significantly improving the overall patient experience. Source Material for Data in Video: https://www.gene.com/media/press-rele...