Скачать с ютуб Galvanic Cell vs Electrolytic Cell animation| Electrochemical Cells Electrochemistry в хорошем качестве
galvanic cell definition
galvanic cell construction
galvanic cell diagram
Galvanic cell working principle
working of galvanic cell
galvanic cell diagram zinc and copper
what is the difference between galvanic cell and electrolytic cell
galvanic cell and electrolytic cell
Galvanic cell and electrolytic cell similarities
galvanic cell and electrolytic cell similarities and differences
digital kemistry
electrochemistry
galvanic cell vs electrolytic cell animation
Скачать бесплатно и смотреть ютуб-видео без блокировок Galvanic Cell vs Electrolytic Cell animation| Electrochemical Cells Electrochemistry в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Galvanic Cell vs Electrolytic Cell animation| Electrochemical Cells Electrochemistry или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Galvanic Cell vs Electrolytic Cell animation| Electrochemical Cells Electrochemistry в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Galvanic Cell vs Electrolytic Cell animation| Electrochemical Cells Electrochemistry
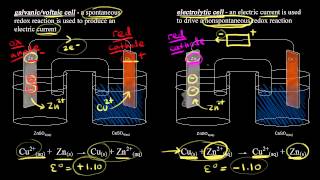
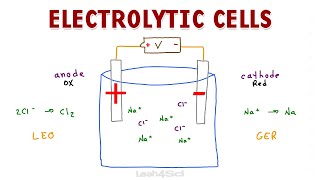
In this video we will discuss the galvanic cell vs electrolytic cell animation,similarities and the differences between the Galvanic cell and Electrolytic cell . Galvanic Cell vs Electrolytic Cell is an important topic of electrochemistry Similarities between galvanic cell and electrolytic cell: Both are electrochemical cells Redox reaction takes place in both cells Both cells contain Anode & Cathode Oxidation takes place at Anode & reduction at Cathode. Difference b/w Galvanic Cell & Electrolytic Cell: Galvanic cell changes chemical energy into Electrical energy . Anode is –ve Cathode is +ve Spontaneous reaction occurs. Does not require external voltage source Electrolytic cell Changes electrical energy into Chemical reaction. Anode is +ve Cathode is -ve Non-Spontaneous reaction occurs. Require external voltage source. ----------------------------------------------- 👉For joining best Chemistry Coaching, Whatsapp number: 03336753424 Location Islamabad, Pakistan Free best Chemistry Notes, 👉 http://mydigitalkemistry.com Subscribe 👉 / digitalkemistry Facebook 👉 / anum.sunum Instagram 👉 / anumsunum 🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️ ✅Buy Now !! ✔Online Original Branded Products in Pakistan at the Lowest Rates 👉 / anum.amazon ✅WhatsApp 03496967013 🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️ For more Chemistry videos: #digitalkemistry #DifferenceBetweenGalvanicandElectrolyticCell #YouTue2021BestChemistryTeacher #youtube2023 #electrochemistryclass12 #chemistrylecture2023