Скачать с ютуб All Possible Reasons for XRD PEAK Shift- Doping, Strains, Annealing and Crystallite Size в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок All Possible Reasons for XRD PEAK Shift- Doping, Strains, Annealing and Crystallite Size в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно All Possible Reasons for XRD PEAK Shift- Doping, Strains, Annealing and Crystallite Size или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон All Possible Reasons for XRD PEAK Shift- Doping, Strains, Annealing and Crystallite Size в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
All Possible Reasons for XRD PEAK Shift- Doping, Strains, Annealing and Crystallite Size
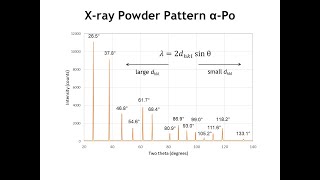
All possible reasons for Peak Shift in XRD! Peak shift in XRD spectrum reveals a lot of information about the material. First observe whether the peak shift is strong or slight! If there is a ‘strong peak displacement’ then calibration, alignment, sample position are the reasons (this is not on our fault, it is related to machine, operator or technician). If there is a ‘slight shift’ then the following factors are responsible! 1- By doping – it changes the stoichiometric composition 2- Lattice strain- macrostrains & microstrains 3- Thermal annealing- 4- Crystallize size - D = Kλ/βcosθ 1- Doping: Introduction of impurities into a crystal lattice can cause to expand or contract the host lattice volume – changes the lattice parameters or produce strain within the material - leads to a significant shift in the XRD peaks. Due to doping, changes in crystal structure, change the lattice parameters, or strain within the material. Here are the main ways doping can cause XRD peak shifts Expansion & contraction of the unit cell due to substitutional doping: Expansion means increasing the lattice parameters (d-value increasing), causes XRD peaks to shift to lower 2θ, while contraction means decreasing the lattice parameters (d-value decreasing) cause peaks to shift to higher 2θ. This can be confirmed by Bragg’s law (λ = 2dsinθ), because the d and 2θ are inversely proportional (d = λ/2dsinθ). This doping only changes the lattice parameters but does NOT change the crystal structure. Expansion & contraction of the unit cell due to substitutional doping: Whereas the interstitial doping causes the same expansion (large d-value) and contraction (small d-value), therefore causing the XRD peak to shift lower 2θ and higher 2θ, respectively. This doping not only changes the lattice parameters BUT also the CRYSTAL STRUCTURE! Doping can cause strain effects i.e., tensile strain- expands the lattice (larger d-value), shifting peaks to lower 2θ while the compressive strain- contracts the lattice (smaller d-value), shifting peaks to higher 2θ. Formation of secondary phases (maybe new peaks appear)- If the concentration of doping is too high, new peaks may appear in the XRD pattern. For example , Al doped ZnO can result in the formation of Al2O3. So, due to the additional phases (Al2O3 in this case), may be some additional peaks appears in the XRD pattern..... 2- Strains Due to MACROSTRAINS in the materials! Peak moves, NO shape changes! d is same throughout the materials! Macrostrains: Let’s suppose the original interatomic spacing is do (shown in the image). If there is uniform tensile strain in the grain at right angles to the reflecting planes, the interatomic spacing (d) becomes larger than do and the corresponding XRD peak (diffraction line) shifts towards the lower 2θ. This is also true from Bragg’s law (λ = 2dsinθ or d =λ/2sinθ) i.e., diffraction angle (2𝜃) is inversely proportional to the interplanar spacing d. 3- Thermal Annealing- Temperature Factor: Due to thermal vibration, even at the absolute zero of temperature, the atoms change its position. The amplitude of vibration increases as the temperature increases thereby the unit cell expands, causing changes in periodicity d-value and therefore changes the positions of the diffraction lines (2θ value). In addition, with rising temperature, the peak intensities of the diffraction lines also decrease while the intensity of background between diffraction peaks increases. Bragg’s law shows that d is inversely proportional to the peak position (2θ value). Therefore, peak shift towards the lower 2θ value. 4- Crystallite Size: Crystallite size can also cause shifts in the positions of diffraction peaks. When the crystallite size decreases, the diffraction peaks tend to shift towards higher 2θ values. This shift is attributed to the increased surface-to-volume ratio in smaller crystallites. As smaller the crystallite size, higher proportion of the atoms are located on the surface or near the surface. These surface or near surface atoms experience different bonding environments as compared to the atoms in the bulk, resulting in modified d-value and therefore, a shift in the diffraction peak positions (2θ value).








![Crystal Parameters-HCP [Year-1]](https://i.ytimg.com/vi/TvRkqL2xid0/mqdefault.jpg)