Скачать с ютуб 8.2 How to Draw Lewis Dot Structures | Complete Guide | General Chemistry в хорошем качестве
lewis structure
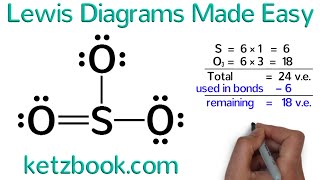
lewis structures
lewis dot structure
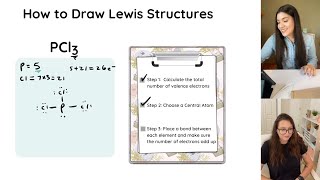
how to draw lewis structures
lewis structure practice problems
lewis dot structure tutorial
drawing lewis structures
lewis diagrams
lewis dot structure practice
lewis dot diagrams
lewis structure chemistry
lewis structure formal charge
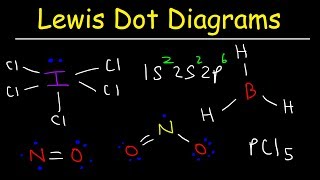
lewis structure for co2
lewis structure of hcn
lewis structure of no3-
expanded octet lewis structure
lewis structure for no3-
drawing lewis structures practice
Скачать бесплатно и смотреть ютуб-видео без блокировок 8.2 How to Draw Lewis Dot Structures | Complete Guide | General Chemistry в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно 8.2 How to Draw Lewis Dot Structures | Complete Guide | General Chemistry или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон 8.2 How to Draw Lewis Dot Structures | Complete Guide | General Chemistry в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
8.2 How to Draw Lewis Dot Structures | Complete Guide | General Chemistry
Chad explains and demonstrates exactly how to draw Lewis Dot Structures in this lesson including numerous examples. The octet rule is introduced and explained followed by the three notable exceptions including expanded octets. How to easily calculate formal charge is also included as well as how to draw multiple resonance structures for certain molecules. It is also shown for molecules exhibiting resonance how to use formal charge to distinguish between individual resonance structures. The Lewis Dot Structures for a large number of molecules are worked out for students including CCl4, NF3, HCN, CO2, N2O, SF4, XeF4, SO4^2-, and NO3^-. I've embedded this playlist as a course on my website with all the lessons organized by chapter in a collapsible menu and much of the content from the study guide included on the page. Check this lesson out at https://www.chadsprep.com/chads-gener... If you want all my study guides, quizzes, final exam reviews, and practice exams, check out my General Chemistry Master Course (free trial available) at https://www.chadsprep.com/genchem-you... 00:00 Lesson Introduction 01:10 Valence Electrons & Lewis Symbols for Elements 03:44 Octet Rule 08:43 Exceptions to the Octet Rule 15:35 CCl4 Lewis Structure 22:01 NF3 Lewis Structure 24:20 HCN Lewis Structure 27:11 CO2 Lewis Structure 34:24 N2O Lewis Structure (Formal Charge) 42:56 SF4 Lewis Structure 46:14 XeF4 Lewis Structure 49:02 SO4^2- Lewis Structure 55:51 NO3^- Lewis Structure (Resonance) https://www.chadsprep.com/ https://courses.chadsprep.com/pages/p...