Скачать с ютуб 9.7 Hydration of Alkynes | Organic Chemistry в хорошем качестве
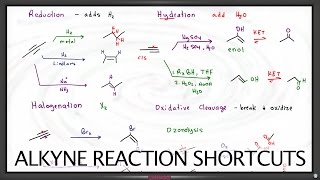
alkyne hydration
alkyne hydration reaction
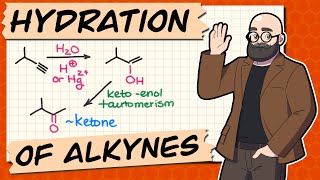
alkyne acid catalyzed hydration
alkyne hydration with mercury
acid catalyzed hydration of alkynes
hydroboration oxidation of alkynes
keto-enol tautomerization
tautomerization mechanism
alkyne
acid-catalyzed tautomerization
hydroboration-oxidation
tautomerization
keto tautomer
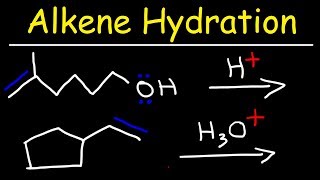
hydration reaction
oxymercuration-reduction
disiamylborane reaction with alkyne
sia2bh reaction with alkyne
sia2bh reaction mechanism
sia2bh reaction
Скачать бесплатно и смотреть ютуб-видео без блокировок 9.7 Hydration of Alkynes | Organic Chemistry в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно 9.7 Hydration of Alkynes | Organic Chemistry или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон 9.7 Hydration of Alkynes | Organic Chemistry в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
9.7 Hydration of Alkynes | Organic Chemistry
Chad covers the Hydration of Alkynes in this lesson. He begins with a review of the three alkene hydration reactions: 1) acid-catalyzed hydration (Markovnikov), 2) oxymercuration-demercuration (Markovnikov), and 3) hydroboration-oxidation (anti-Markovnikov). He then relates to these three alkene reactions the two alkyne hydration reactions: 1) acid-catalyzed hydration of alkynes (Markovnikov) and 2) hydroboration-oxidation of alkynes (anti-Markovnikov). He shows that the addition of one equivalent of H2O across the alkyne results in an enol which tautomerizes to either a ketone or an aldehyde. He also shows that it is common with alkynes to use a bulky borane for hydroboration oxidation such as disiamylborane (Sia2BH) or dicyclohexylborane. Chad concludes the lesson by showing the mechanisms for Keto-Enol Tautomerization, both acid-catalyzed and base-catalyzed which commonly appear on undergraduate exams covering the alkyne material. If you want all my study guides, quizzes, and practice exams, check out my premium course at https://www.chadsprep.com/organic-che... 00:00 Lesson Introduction 00:33 Review of the Hydration of Alkenes 01:40 Acid-Catalyzed Hydration of Alkynes (Markovnikov) 05:56 Hydroboration-Oxidation of Alkynes with disiamylborane (anti-Markovnikov) 09:36 Hydration of Internal Alkynes 11:33 Keto-Enol Tautomerization 13:22 Keto-Enol Tautomerization Mechanism (Acid-Catalyzed) 14:37 Keto-Enol Tautomerization Mechanism (Base-Catalyzed) https://www.chadsprep.com/