Скачать с ютуб The 5 most relevant changes the Medical Device Regulation MDR introduces, that you must know в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок The 5 most relevant changes the Medical Device Regulation MDR introduces, that you must know в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно The 5 most relevant changes the Medical Device Regulation MDR introduces, that you must know или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон The 5 most relevant changes the Medical Device Regulation MDR introduces, that you must know в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
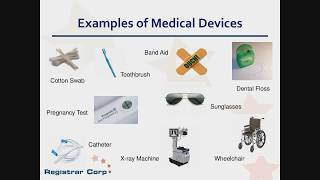
The 5 most relevant changes the Medical Device Regulation MDR introduces, that you must know
The Medical Device Regulation MDR replaces both, the Medical Device Directive (MDD, 93/42/EEC) and the Directive for Active Implantable Medical Devices (AIMDD). This video gives an overview on the 5 most relevant changes the MDR introduces. These changes include 1. Conformity assessment procedures 2. Classification 3. Essential requirements - General safety and performance requirements 4. UDI & EUDAMED 5. Post-Market requirements The Johner Institute helps medical device manufacturers to fast and easily navigate the approval processes, to fulfill the MDR requirements and to obtain the CE mark. Complimentary consulting on www.johner-institute.com.