Скачать с ютуб structural changes & SAR for lead optimization в хорошем качестве
Chem Help ASAP
Chem Help
Chem ASAP
ASAP chem
medicinal chemistry
drug discovery
pharmacokinetics
lead optimization
hydrogen bond
SAR
structure activity relationship
structure-activity
structure activity
potency
efficacy
safety
ADME
PK
relationship
structure
drug structure
drug SAR
SAR drug
hERG inhibition
hERG
drug drug interaction
drug interaction
drug-drug interaction
DDI
solubility
membrane permeability
metabolism
drug metabolism
Скачать бесплатно и смотреть ютуб-видео без блокировок structural changes & SAR for lead optimization в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно structural changes & SAR for lead optimization или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон structural changes & SAR for lead optimization в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
structural changes & SAR for lead optimization
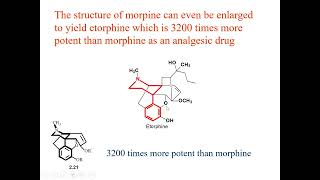
directory of Chem Help ASAP videos: https://www.chemhelpasap.com/youtube/ The fundamental idea behind lead optimization is that to change (hopefully improve) a molecule’s activity, one needs to change a molecule’s structure. This is the crux of SAR – structure-activity relationships. In terms of activity, we can discuss potency and efficacy, which typically trend together, ADME & PK, and safety. There is no single, universal method for picking which groups to test in a lead series to optimize a structure, but this video presents some commonly used approaches. If the discovery group is fortunate enough to have structural information on the target (for example, an x-ray structure), then the group will use the target structure to guide the addition of lipophilic groups to increase hydrophobic interactions. Lipophilic groups include small alkyl chains and halogen atoms. If longer chains are beneficial, then maybe entire phenyl groups will be explored as well. Hydrogen bond donors (OH and NH groups from alcohols, amines, and amides) or hydrogen bond acceptors (almost any oxygen or nitrogen with a lone pair) can be added to create hydrogen bonding interactions with the backbone of the target peptide or a side chain of an amino acid residue in the target. What if you don’t have a structural model of the target? You use the same groups, but changes involve much more trial and error. Optimizing ADME and PK involves a range of properties. Solubility can be improved by adding polar or charged groups. Changes are normally made away from key pharmacophore features so that potency is not reduced. Leads are often substrates of efflux transporters, which can reduce absorption and oral bioavailability. Reducing lipophilicity of a compound can reduce efflux, so adding polar groups or increasing polar surface area is one possible solution. Finally, compound with a short half-life due to high hepatic clearance may be “hardened” or made more resistant to metabolism through replacing a C-H with a C-F at the site of metabolism, especially on aromatic rings. The electronegative fluorine makes the ring less prone to oxidative metabolism. A common safety risk for leads is hERG inhibition, which is often seen in lipophilic amines. Many drugs are lipophilic amines. Adding groups to increase polarity or polar surface area can reduce hERG channel inhibition. Similarly, adding groups that reduce amine basicity, such as adding a nearby electronegative atom, can also be beneficial. Another common safety risk is inhibition of one or more cytochrome P450 enzymes, which can lead to drug-drug interactions. Inhibition is often linked to pyridine-type nitrogens, which can bind the heme iron in the active site of the enzyme. Introducing a steric group, even just a methyl, next to the nitrogen can reduce inhibition.






![1 A.M Study Session 📚 [lofi hip hop]](https://i.ytimg.com/vi/lTRiuFIWV54/mqdefault.jpg)