Скачать с ютуб Serial dilutions в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Serial dilutions в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Serial dilutions или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Serial dilutions в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
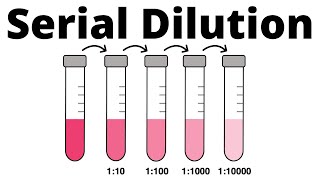
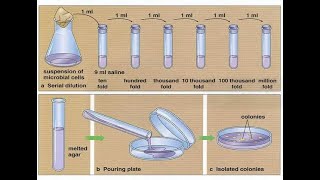
Serial dilutions
Take a spoonful of Cheerios and add it to a spoonful of milk, stir it up well, take a spoonful of this new mix and add it to a spoonful of fresh milk, stir that up & add a spoonful of it to another spoonful of new milk and… You’ve got yourself a SERIAL cereal DILUTION! Which I have to admit would have been a lot more fun than the actual serial dilutions I spent part of my morning doing… blog form: http://bit.ly/serialdilutions note: text old, video new We’ve looked at a lot of “glamour” experiments - and that’s the type of stuff you see in the news too - how CRISPR gene editing is being used to treat patients with sickle cell disease, how scientists are determining the atomic structures of key biological molecules, etc. What you don’t usually hear about is all the hard, less-glamorous, work that goes into those discoveries. A lot of day-to-day lab life isn’t “glamorous” in any sense of the word, but it’s still important - and doesn’t have to be boring. And, sometimes if you make seemingly boring tasks more fun you can come to appreciate them more, understand them at the molecular level, and even perform them better. One such task is the serial dilution. In a serial dilution you perform a series of stepwise dilutions where you’re diluting by the same factor each time (e.g. 1:2 in this cheerio-y example), so the concentration (relative amount of the thing to the non-thing) decreases “exponentially.” We can put some terminology on it and say that each mix has a Dilution-Factor-times less than the one before it. So in this case, our Dilution Factor (DF) is 2, each new Cheerio mix has 1/2 as many Cheerios per spoonful than the one before it. If instead of mixing 1 spoonful to 1 spoonful each time we’d mixed 1 spoonful to 9 spoonfuls of milk (a 1:10 dilution) our Cheerios would dilute out a lot faster. We’d have a Dilution Factor of 10, so each new spoonful would have 10-times-less Cheerios than the one before. Serial dilutions are kinda like pyramid schemes with the opposite effect - if you start out with a certain amount of money it gets distributed to more and more people - but you can’t give out more money than you started with so people end up with less and less money. When we have something changing by a constant multiplier (like 1/2 or 1/10) we call it geometric, or exponential, growth/decay. And it gives us shortcuts to work with as we’ll see… So, how do serial dilutions work & why do we use them? You keep diluting the same thing over and over but in a constant-stepwise fashion, stepwise being key & they’re useful for lots of things - like helping with accuracy & creating “standard curves” which are a series of things of known concentration that allow you to compare yours to. More on these why’s after we discuss the how But let’s get back to the how for now, skipping the part where you milk the cow - say you want to do 1:2 dilutions of Cheerios, we’ll call them H for shortness, since C will mean something else (concentration) later and I don’t want anyone to get confused. So take the starting solution, which is all H. And you mix it with an equal amount of milk. e.g. if you took 1 spoonful of H + 1 spoonful of milk you’d get 2 spoonfuls of of a 50% H solution Then you take that 1st dilution and do the same thing with it - but now since you’re starting with a 50% solution, when you have it, you get a 25% solution. And if you do the same thing with the 25% solution, you’ll get a 25/2= 12.5% solution, and you can keep doing this, each time getting a solution half as dilute as the one before. 100% → 50% → 25% → 12.5% → 6.25% So in the span of 5 samples you can cover a spread from 100% to 6.25% If that’s not big enough for you, you can keep on diluting (e.g 6.25% → 3.125%…) But you’ll still only be decreasing by 1/2 each time. If you want to “move faster” you can choose a higher dilution factor. Dilution factor (DF) refers to the amount by which you’re diluting by each step. So in this example, the dilution factor was 2/1 = 2. What if instead of 2 you chose a dilution factor of 10? Now over the span of 5 samples you go from 100% → 10% → 1% → 0.1% → 0.01% So, for example, if you started with 100 spoonfuls of Cheerios (H), the 5th tube would have 1 spoonful-worth of Cheerios and 99 spoonfuls-worth of milk. But you didn’t get there by mixing 1 spoonful of Cheerios with 99 spoonfuls of milk - you got there stepwise. solution 1) 100 H solution 2) 10 H + 90 milk solution 3) 10(10 H + 90 milk) + 90 milk solution 4) 10(10(10 H + 90 milk) + 90 milk) solution 5) 10(10(10(10 H + 90 milk) + 90 milk)) + 90 milk finished in comments