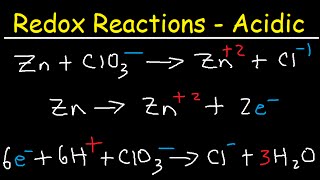Скачать с ютуб How to Balance Redox Equations in Acidic Solution в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок How to Balance Redox Equations in Acidic Solution в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно How to Balance Redox Equations in Acidic Solution или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон How to Balance Redox Equations in Acidic Solution в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
How to Balance Redox Equations in Acidic Solution
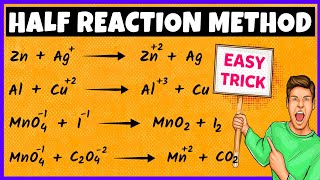
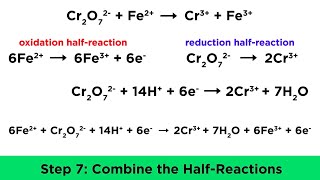
We'll go step by step through how to balance an oxidation reduction (redox) reaction in acidic solution. Most importantly, both charges and atoms must balance. Here are the steps: first, calculate oxidations numbers for all the elements in the equation. Next, figure out what is being oxidized and what is being reduced. Then, write half reactions for the oxidation and reduction. After that, balance each half reaction: first, for the atoms other than O and H, then for O and H, and finally for charge by adding electrons. After being balanced, the oxidation and reduction half reactions are ready to be added back to together. Make sure that the number of electrons is the same in the oxidation and reduction half reactions. If they are not, multiply one or both of the half reactions to make the number of electrons the same. Then, combine the oxidation and reduction half reaction, canceling out stuff that appears on both sides of the equation. Lastly, do a final check to make sure that everything balances: both atoms and charge.