Скачать с ютуб Polarity & Solubility of Benzoic acid в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Polarity & Solubility of Benzoic acid в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Polarity & Solubility of Benzoic acid или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Polarity & Solubility of Benzoic acid в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
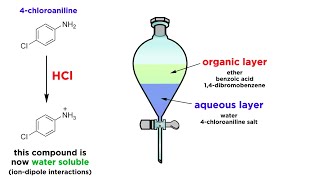
Polarity & Solubility of Benzoic acid
The question is whether C6H5COOH (Benzoic acid) soluble or insoluble in water? We'll also look at the polarity for the Benzoic acid molecule at well to help us understand whether it is soluble or insoluble. The answer is that C6H5COOH is not very soluble in water at normal temperature. It has a large nonpolar section of the molecule (the ring) which does not dissolve in water. At the same time it does have the polar carboxyl group -COOH that is polar and interacts with water. Because of this it's solubility at lower temperatures is limited (although it is more soluble in water at higher temperatures). Solubility of Benzoic acid (from https://en.wikipedia.org/wiki/Benzoic...) 1.7 g/L (0 °C) 2.7 g/L (18 °C) 3.44 g/L (25 °C) 5.51 g/L (40 °C) 21.45 g/L (75 °C) 56.31 g/L (100 °C) More Learning Resources • Polar and Non-Polar Tutorial: • Polar, Non-Polar, and Ionic Compounds... • Drawing Lewis Structures: • Lewis Structures for Covalent Molecul... • Determining Molecular Geometry: • Molecular Geometry: Rules, Examples, ... • Periodic Table with Electronegativity Values: https://en.wikipedia.org/wiki/Electro... (data_page) • Molecular Geometry App: https://phet.colorado.edu/sims/html/m... Molecular Visualization Software: https://molview.org/ Chemistry help at https://www.Breslyn.org For ionic compounds, unlike Carbon tetrachloride, we can use these basic rules for solubility (see • Solubility Rules: Explanation & Prac... ): Salts of: - Group I elements (Li+, Na+, K+, Cs+, Rb+) are - NH4+ (Ammonium ion) are soluble. - the nitrate ion (NO3-) are generally soluble. - of Cl-, Br-, and I- are soluble. Exceptions Ag+, Pb2+, and (Hg2)2+ - most sulfates are soluble. Exceptions: Ba2+, Ca2+, Pb2+, Ag+, Sr2+ - most hydroxide salts are only slightly soluble. Exceptions: NH4+, Li+, Na+, K+ - most carbonates (CO32-) are insoluble. Exceptions: Group 1 and NH4+ Note: Rules at the top supersede any lower rules.