Скачать с ютуб Pathogen Recognition Receptors & Innate immune Response || Toll-like Receptors в хорошем качестве
toll like receptors
toll like receptor
pathogen recognition receptors
immunology animation
TLR
innate immunity
innate immune response
pattern recognition receptors innate immunity
c-type lectin receptors (clr)
dendritic cells
toll-like receptors (tlr)
nod like receptors
pathogen-associated molecular patterns (pamps)
immunology introduction
endotoxin
neutrophils and epithelial cells
inflammation
immunology lecture
flagellin
macrophages
lipopolysachharide
Скачать бесплатно и смотреть ютуб-видео без блокировок Pathogen Recognition Receptors & Innate immune Response || Toll-like Receptors в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Pathogen Recognition Receptors & Innate immune Response || Toll-like Receptors или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Pathogen Recognition Receptors & Innate immune Response || Toll-like Receptors в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Pathogen Recognition Receptors & Innate immune Response || Toll-like Receptors
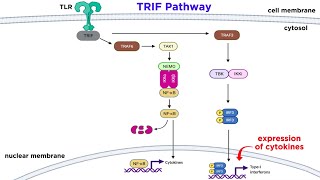
#immunology #tolllikereceptor #virus In the early phases of an immune response, the innate immune system detects pathogens and acts as the first line of defence. Dendritic cells, which circulate throughout tissues, can detect the presence of pathogen-associated molecular patterns, or PAMPs. PAMPS are pathogen traits that are conserved, such as lipopolysaccharides (LPS), which are components of the cell membranes of all gram-negative bacteria. Dendritic cells can recognise PAMPs by expressing a family of Toll-like receptors, also known as TLRs. In the case of LPS, it is identified by TLR-4, a member of the TLR family expressed on the surface of dendritic cells. LPS is carried to the dendritic cell surface by the soluble LPS-binding protein, LBP, and deposited on the cell surface protein CD14. TLR-4 detects the presence of LPS by interacting with and recognising LPS bound to CD14. The signal produced by the TLR stimulates dendritic cell maturation. At this point, the dendritic cell can move to regional lymph nodes and activate the acquired immune response. Immune system cells, such as macrophages and dendritic cells, serve as the first line of defence in identifying pathogens of various types. These cells have evolved a variety of receptors for identifying various pathogen-associated molecular patterns (PAMPs). These proteins are divided into groups that identify various types of PAMPs. Toll-like receptors, or TLRs, are made up of numerous leucine-rich repeats that help TLRs recognise different PAMPs. TLRs are membrane-associated proteins. Some are on the cell's surface, while others are on endocytic vesicles, where they check the degraded contents of pathogens picked up by endocytosis. Different types of PAMPs are recognised by each member of the TLR family. TLR-5, for example, identifies flagellin, a highly conserved component of the bacterial flagellum. Bacterial genomes include methylated CpG oligonucleotide patterns that TLR-9 recognises after the genome is destroyed in the lysosome. TLR-6 and TLR-2 form a dimer that detects diacyl lipopeptides; TLR-1 and TLR-2 form a dimer that recognises triacyl lipopeptides; and TLR-4 recognises lipopolysaccharide, or LPS, a gram-negative bacterium component. TLR-3 and TLR-7, like TLR-9, are found on endocytic vesicles and identify double-stranded and single-stranded RNA, respectively. When any TLR is triggered, transcription factors are activated, which sends a signal to the nucleus. However, not all infections live in the extracellular space or are phagocytosed. Viruses and other pathogens live and proliferate in the cytosol. There are at least two types of receptors that can detect infections in the cytosol and alert the immune system to their presence. Members of the Nucleotide Oligomerization Domain family, or NOD proteins, are one type of such receptor. The cytosolic NOD2 protein, for example, may detect bacterial proteoglycans of intracellular bacteria. When the NOD2 protein identifies its ligand, muramyl dipeptide, it sends a signal to the nucleus, causing transcription to begin. Finally, an RNA helicase domain and two caspase recruitment domains, or CARD domains, are found in a class of intracellular receptor proteins. RIG-I, a member of this family, identifies double-stranded RNAs, which are involved in the life cycle of many RNA viruses. This protein class, like TLRs and NODs, delivers a signal to the nucleus, but unlike TLRs and NODs, it triggers the synthesis of Type-1 interpherons. Toll-like receptors, NOD proteins, and the RNA helicase CARD domain family all enable the innate immune system to recognise external and intracellular pathogens and mount an immune response against them.